Abstract
Anti-CD38 therapeutic monoclonal antibodies (mAbs) such as daratumumab (dara) have shown wide application and therapeutic benefit for Multiple Myeloma (MM) patients. However, antibody-dependent cell-mediated cytotoxicity (ADCC), a critical mechanism of action associated with many mAbs, is not realized for anti-CD38 mAbs as the effector cells with the potential to elicit ADCC, namely natural killer (NK) cells, are also positive for CD38 surface expression and succumb to anti-CD38 mAb-mediated fratricide. To develop a therapeutic strategy that captures the full breadth of the anti-tumor capacity of dara and other anti-CD38 mAbs, we utilized our induced pluripotent stem cell (iPSC) platform, where the generation of precisely engineered master iPSC lines serve as the starting material for the manufacture of uniformly engineered immune cells for off-the-shelf cell therapies. To generate an iPSC-derived NK (iNK) cell capable of maximizing ADCC in combination with dara, a master iPSC line (termed FT538) was created containing three unique edits; a high-affinity, non-cleavable CD16 (hnCD16) to potentiate ADCC in combination with mAb, a recombinant IL-15 receptor fusion (IL-15RF) for cytokine-autonomous persistence and importantly, deletion of CD38 expression to prevent anti-CD38 mAb mediated fratricide and to enhance NK cell fitness. In addition to previous studies, we show here in a variety of preclinical models, FT538 has the unique ability to exhibit potent ADCC when combined with dara by avoiding anti-CD38 mAb-mediated fratricide (MM1s tumor remaining after FT538 + dara = 16%, after primary NK cell + dara = 61%). Similar results were seen in xenograft tumor models of MM, where established MM1S cells were controlled for growth when dara was combined with FT538 compared to dara alone (p<0.001) or FT538 alone (p<0.001).
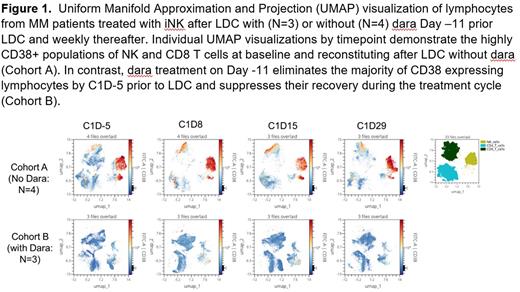
Having established the unique ability of FT538 to avoid fratricide in the presence of dara, we next investigated whether anti-CD38 mAb could also deplete alloreactive CD38+ lymphocytes to reduce or eliminate the need to administer the unfavorable lymphodepleting conditioning (LDC) currently used in cellular immunotherapy. To evaluate the effect of anti-CD38 mAb on host lymphocyte reconstitution we used flow cytometry to characterize PBMCs or marrow collected from MM patients after LDC with cytoxan 300 mg/m2 and fludarabine 30 mg/m2 x 3 days either as monotherapy (N = 4) or in combination with dara 16 mg/kg 1 week prior to LDC (Day -11) and weekly thereafter (n = 3) (NCT05182073). Prior to LDC the NK cell population in patients without dara treatment was highly CD38+ (80-90%). However, after 1 dose of dara patient NK cells expressed reduced CD38 (all <20%). In monotherapy patients CD8+ T cells reconstituting after LDC were predominantly CD38+ by Day 8 (80-90%) and remained so through Day 29 (Figure 1). In contrast, in dara treated patients reconstituting NK and T cells were significantly reduced in number with low CD38 expression. The same pattern was observed in bone marrow lymphocytes.
To fully eliminate the dependency on LDC, FT538 was further engineered with the knockout of beta-2 macroglobulin and class II major histocompatibility complex transactivator (B2M/CIITA-null) to render it undetectable by CD8+ cytotoxic and CD4+ T cells, respectively. B2M/CIITA-null FT538 showed complete loss of HLA class I and class II expression, became unrecognizable by CD8+ and CD4+ T cells and in combination with dara, displayed persistence in various allogeneic models consisting of co-culture with allogeneic NK and T cells. In an in vivo allogeneic model, B2M/CIITA-null FT538 and allogeneic NK cells were co-infused into NOG-IL15 mice with or without anti-CD38 mAb. In the presence of anti-CD38 mAb treatment, B2M/CIITA-null FT538 continued to persist while allogeneic NK cells were not detected, while in the study arm that lacked anti-CD38 mAb, B2M/CIITA-null FT538 was eliminated by the persisting allogeneic NK cells (p<0.001).
In summary, these data demonstrate the potential to combine off-the-shelf iPSC-derived CD38-null NK cells with anti-CD38 mAb as a novel therapeutic strategy, uniquely demonstrating the potentiating of ADCC while reducing or eliminating the need for LDC in cell therapy.
Disclosures
Mbofung:Fate Therapeutics Inc: Current Employment. Zong:Fate Therapeutics: Current Employment. Varady:Fate Therapeutics: Current Employment. Williams:Fate Therapeutics: Current Employment. Hayama:Fate Therapeutics: Current Employment. Pan:Fate Therapeutics: Current Employment. Devkota:Fate Therapeutics: Current Employment. Groff:Fate Therapeutics: Current Employment. Meza:Fate Therapeutics: Current Employment. Dailey:Fate Therapeutics: Current Employment. Lee:Fate Therapeutics: Current Employment. Wong:Fate Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company; Bristol Myers Squibb: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Cooley:Fate Therapeutics, Inc.: Current Employment. Bjordahl:Fate Therapeutics: Current Employment. Goodridge:Fate Therapeutics: Current Employment. Valamehr:Fate Therapeutics: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal